What is emissivity?
All materials absorb, reflect and emit radiant energy, and emissivity is the measurement of how well the surface of a particular material emits heat in the form of infrared energy.
How does it work?
The measurement for emissivity is expressed as a value between zero and one. It represents the ratio between the energy radiated from the material’s surface, and the energy radiated from another material referred to as a blackbody.
A blackbody absorbs thermal radiation and emits it so well, it’s the standard to which all other surfaces are compared.
The blackbody’s emissivity rating is the highest possible, at 1. At the other end of the scale, the more reflective the surface, the lower its emissivity – so a perfect reflector would have a rating of zero.
Shiny polished brass, for example, has an emissivity value of about 0.03. Brick, on the other hand, with its duller and much less reflective surface, has a higher emissivity value of approximately 0.86.
However, bear in mind that in reality there’s no such thing as a material with a perfect zero rating, or a material with a perfect one rating – they’re both theoretical and serve to define each end of the scale.
In real terms, though, the images here are of a Leslie Cube – a stainless steel cube which is filled with hot water to demonstrate how the emissivity of different surfaces can differ, even if they’re all at the exact same temperature.
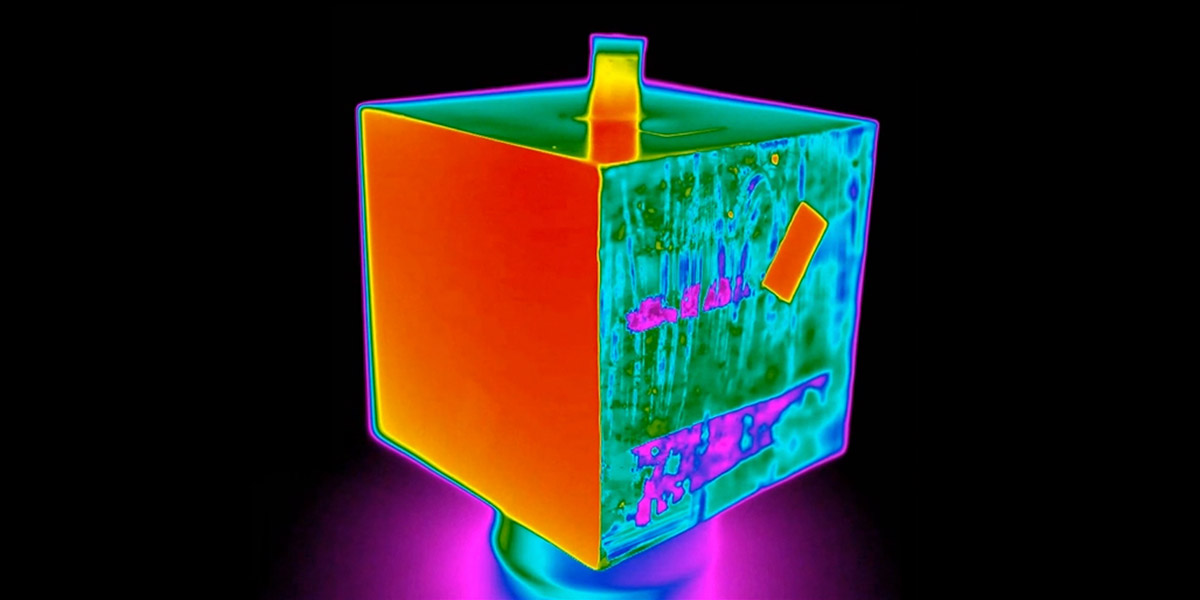
A Leslie Cube filled with warm water
The image above shows two sides of a Leslie Cube. The first, on the left, is painted white with a small piece of electrical tape, On the right, the material is much more shiny and reflective, meaning it has a low emissivity (the darker, purple colouring in the thermal image). On this side, there is also a piece of electrical tape which has a higher emissivity, indicated by the brighter colour.
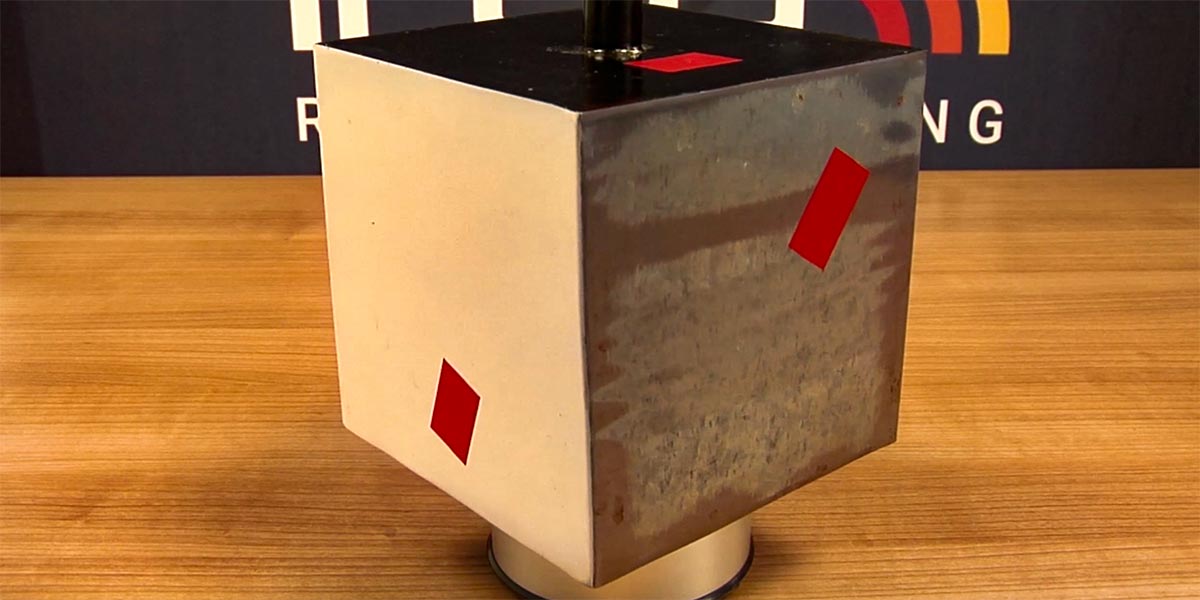
A digital image of a Leslie Cube
As you can see from the digital image, the shinier side also has a more abrasive surface, which distorts the surface temperature of the cube.
As materials with a low emissivity are more reflective, they reflect more ambient radiation than emitted radiation. Because of this, when viewed through a thermal imaging camera, materials warmer than their environment will appear cooler than they should, and when cooler than their environment they will appear warmer.
In this instance, to measure the true temperature of the cube, a thermographer would have to adjust the camera settings to match the emissivity value for a specific surface finish (such as the tape), and measure that surface’s temperature value.
A practical example of Emissivity
One good example of the practical use of emissivity would be the foil-blanket (space blanket) wrapped around a runner at the end of a marathon.
The low emissivity surface on the inside of the blanket means that it reflects most of the heat generated by the runner’s body, reflecting it back to warm up the runner.
If that inside surface had a higher emissivity, it would be more likely to absorb the heat, making the blanket less effective. For example, instead of sending it back where it needs to go, the blanket would absorb some of the heat and subsequently emit it outside of the blanket.
When it comes to thermal imaging of any kind, you need to be aware of emissivity values to make sure your temperature measurements and other related calculations are accurate.
There are ways of estimating those values, either with emissivity lookup tables, or by comparing the surface in question with an object with a known emissivity value.
Once you have those values, you can then adjust your thermal imaging camera to create the most accurate thermal image for your analysis of the situation.

